10 2021 at 619 AM CDT Updated. The Food and Drug Administration approved the Pfizer-BioNTech COVID-19 vaccine for 12- to 15-year-olds in the US.

Pfizer Covid Vaccine Fda Clears Use In Kids Ages 12 To 15
Food and Drug Administration has expanded the emergency use authorization for the Pfizer-BioNTech Covid-19 vaccine.
Fda approved covid vaccine 12-15. May 3 Reuters - The US. Food and Drug Administration is preparing to authorize Pfizer Inc and German partner BioNTech SEs COVID-19 vaccine. The Pfizer-BioNTech vaccine was authorized for use in people ages 16 and up in December.
10 2021 at 620 AM CDT. Pfizer and German partner BioNTech were cleared last month to use their vaccine for 12-to-15-year-olds. Now that the US.
The FDA has approved the Pfizer Covid-19 vaccine for children 12-years-old and up a move that could make millions more shots available. Former pandemic and emerging threats coordinator under President Obama Dr. The FDAfirst gave this vaccine emergency use authorization for people age 16 and older in late 2020.
Food and Drug Administration expanded the emergency use authorization EUA for the Pfizer-BioNTech COVID-19 Vaccine for the prevention of coronavirus disease 2019 COVID-19 caused. Younger children may not have access to the vaccines until early fall. Side Show Stock iStock The Food and Drug Administration FDA late yesterday expanded the emergency authorization of the Pfizer COVID-19 vaccine to include 12- to 15-year-olds paving the way for vaccinating a proportion of school-age children before the fall.
A doctor prepares to administer a vaccine injection at New York-Presbyterian Lawrence Hospital in Bronxville NY in January. The Food and Drug Administration FDA has approved the Pfizer-BioNtech COVID-19 vaccine for use in children aged 12 to 15 years of age. The Pfizer-BioNTech COVID-19vaccine requires two injections given 21 days apart.
On Monday the FDA amended its previous emergency use authorization for the Pfizer vaccine which only allowed inoculation of those 16 and up to include the younger age group. 104 rânduri The FDA expanded the emergency use authorization for the Pfizer-BioNTech COVID-19 Vaccine to include 12 15 year olds and issued an updated FDA COVID-19 Response At-A-Glance Summary. Food and Drug Administration FDA has given the Pfizer-BioNTech COVID-19vaccine emergency use authorization for children ages 12 through 15.
Both Moderna and Pfizer-BioNTech launched trials of their Covid-19 vaccines for kids under 12 in March. The Food and Drug Administration has approved. The first to be authorized for children under age 16.
The FDA has now amended the authorization to include children ages 12. They got an emergency use authorization for this age group in May. However the COVID-19 vaccines are currently only approved in the United States for people 12 years and older.
We are pleased to announce that we have submitted for. This announcement of Emergency Use Authorization EUA was made on Monday May 10 and signals a major step forward in the national effort to get every citizen inoculated. FDA may authorize Pfizers COVID-19 vaccine for 12 to 15-year-olds this week By Cali Hubbard Published.
Results are expected in the fall and it will take FDA officials time to review the drug. Mario Ramirez joins News NOW to explain some parents safety concerns and what the FDAs decision could mean for achieving herd immunity. The companies said they plan to request approval for people ages 12 to 15 once they have additional required data.
In a news briefing Monday evening after the announcement FDA officials said the Pfizer authorization for 12- to 15-year-olds was a straightforward decision because the data showed that the. Food and Drug Administration is aiming to give full approval to Pfizers COVID-19 vaccine by early September according to The New York Times. The FDA approved the use for that age group on Monday.
Food and Drug Administration has authorized emergency use of Pfizers COVID-19 vaccine for children ages 12 to 15 Monday what.
Some Parents Still Skeptical As Fda Authorizes Pfizer Vaccine For Children Ages 12 15 Video Abc News

Pfizer Asks Us To Allow More Kids To Get Vaccine Katv

Fda Set To Ok Pfizer Covid 19 Vaccine For Ages 12 15 By Next Week Manila Bulletin
Covid 19 Vaccine In The Us News Summary 7 May 2021 As Com
Moderna Asks Fda For Adolescent Covid Vaccine Approval
Special Report The Ex Pfizer Scientist Who Became An Anti Vax Hero Reuters

Covid 19 Vaccine For 12 To 15 Year Olds What Parents Should Know Nebraska Methodist Health System
Fda Reportedly Plans To Authorize Pfizer S Covid Vaccine For Teens 12 15 In Days Ars Technica

Fda Amends Pfizer Vaccine Eua To Include Children Aged 12 15
Get To Know The Covid 19 Vaccines Health Mil
Fda Oks Pfizer Covid 19 Vaccine For 12 15 Age Group Ktep
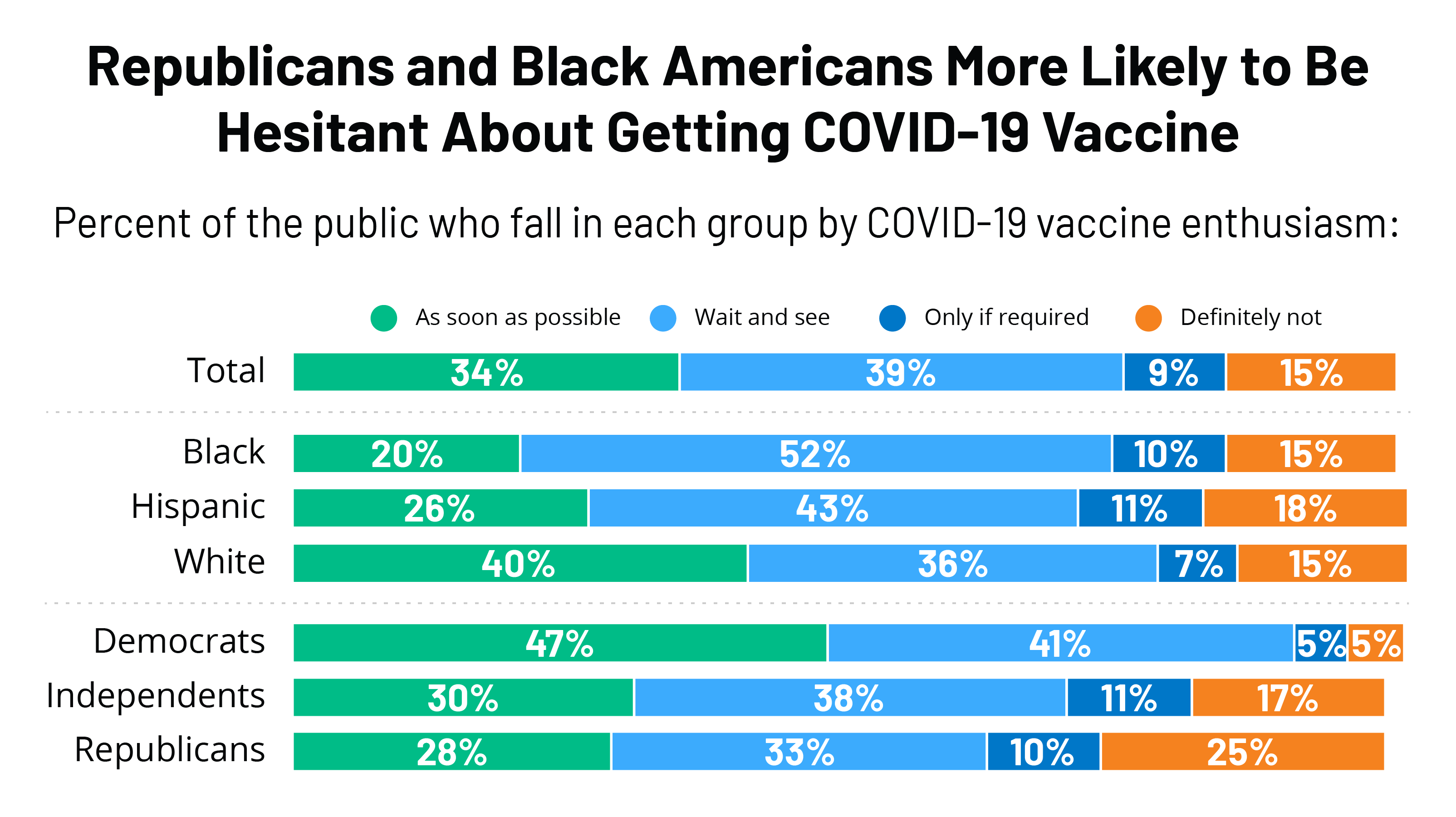
Kff Covid 19 Vaccine Monitor December 2020 Kff
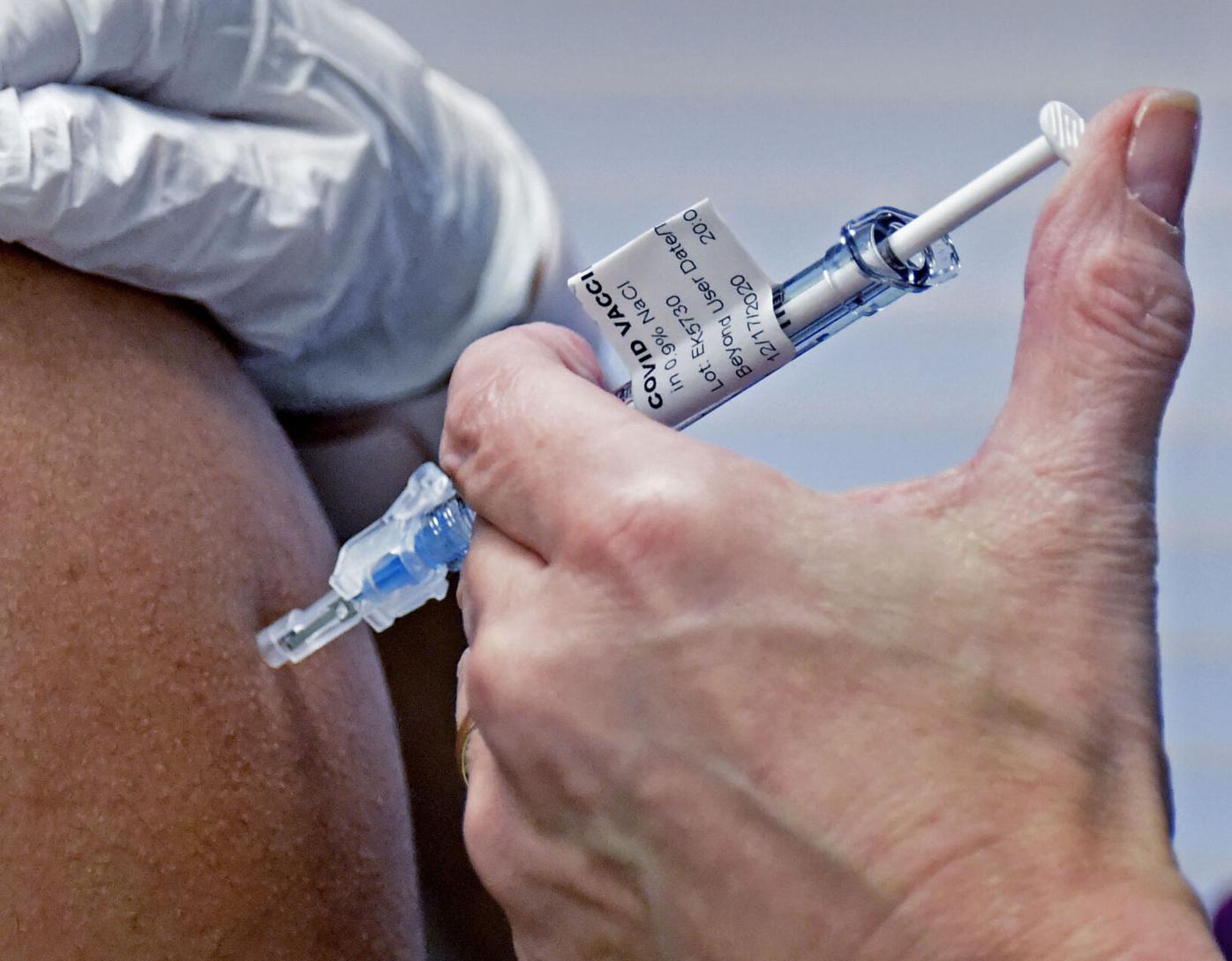
What The Fda Approval Means For Vaccinating Children Ages 12 To 15 Health Lancasteronline Com

Pfizer Covid Vaccine Fda Clears Use In Kids Ages 12 To 15

Pfizer Covid Vaccine Authorized For Ages 12 15 By Fda Localmemphis Com
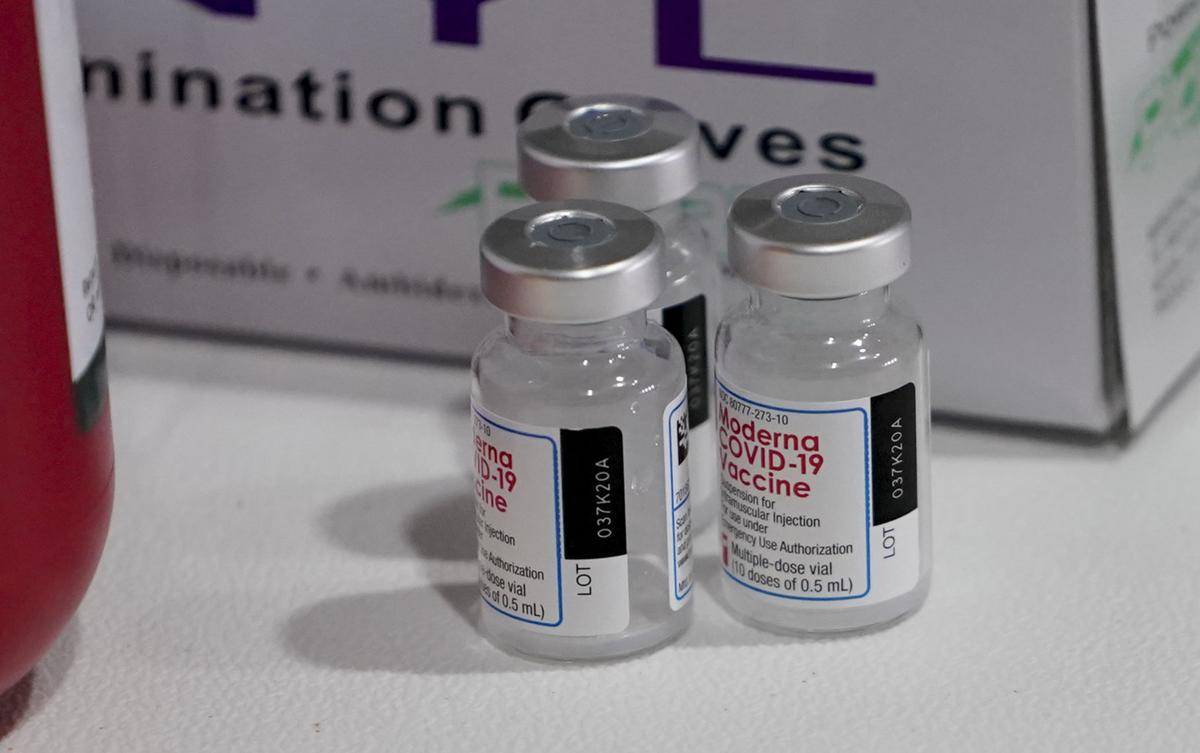
Pfizer Covid 19 Vaccine Approved For Age 12 15 By Fda Wait List Open In Juneau County Regional News Wiscnews Com

Fda Gives Full Approval To Pfizer Covid 19 Vaccine

Pfizer Vaccine For 12 15 When Will Kids Get Covid Vaccine Weareiowa Com


